The Binding Bias: How Organoids Are Redefining What Matters in ADC Efficacy
Published by HUB Organoids on Oct 13, 2025

Antibody–drug conjugates (ADCs) are designed to bring the precision of targeted therapy together with the potency of cytotoxic payloads. Naturally, much of the focus during ADC development has been on binding - ensuring that the antibody component efficiently and specifically recognizes its antigen target.
In fact, in a recent survey we conducted, nearly 40% of participants said that binding is the most important feature when developing an ADC. This makes intuitive sense: after all, an ADC can only deliver its payload if it first binds to the target cell.
Results from our recent survey that shows 40% of the participants said antigen
specificity/binding is the most difficult ADC feature when developing an ADC
However, emerging evidence, including our own organoid data, shows that binding alone doesn’t predict clinical response. The assumption that “more target equals better efficacy” is increasingly being challenged.

POSTER
Patient-derived organoids as a reliable screening platform for assessing ADC efficacy and specificity
The missing link between antigen binding and response
When we tested Tivdak™ (Tisotumab Vedotin) - an ADC that targets CD142 (Tissue Factor) across multiple cancer organoids, we saw a different story. Tivdak had already shown promise in cervical cancer, yet even in the clinic, it was clear that not every patient with high CD142 expression responded. That inconsistency sparked an important question: Was binding really the driving factor behind response, was something else at play?
To find out, we turned to our biobank of patient-derived organoids (PDOs), representing a diverse set of 22 tumor models across multiple cancer types — including bladder, lung, colon, breast, pancreas, head and neck, and ovarian cancers. Each organoid model retained the genetic and phenotypic complexity of the original patient tumor, making it an ideal system to explore this question.
Study design testing Tivdak™ in 22 patient-derived organoids across bladder, lung, colon, breast, pancreas, head & neck, and ovarian cancer.
We first measured CD142 expression in all organoids, using both flow cytometry analysis and RNA sequencing. The two data sets lined up beautifully - a reassuring sign that our expression data were solid. But when we compared those expression levels to how each organoid actually responded to Tivdak, the story changed completely.

CD142 flow cytometry expression correlates with RNAseq data
What we saw was striking: tumors with high CD142 expression didn’t necessarily respond better, and some models with only modest expression showed strong sensitivity. In other words, binding alone didn’t explain efficacy. This pattern echoed what clinicians had observed in cervical cancer patients — a clear disconnect between target expression and treatment outcome.
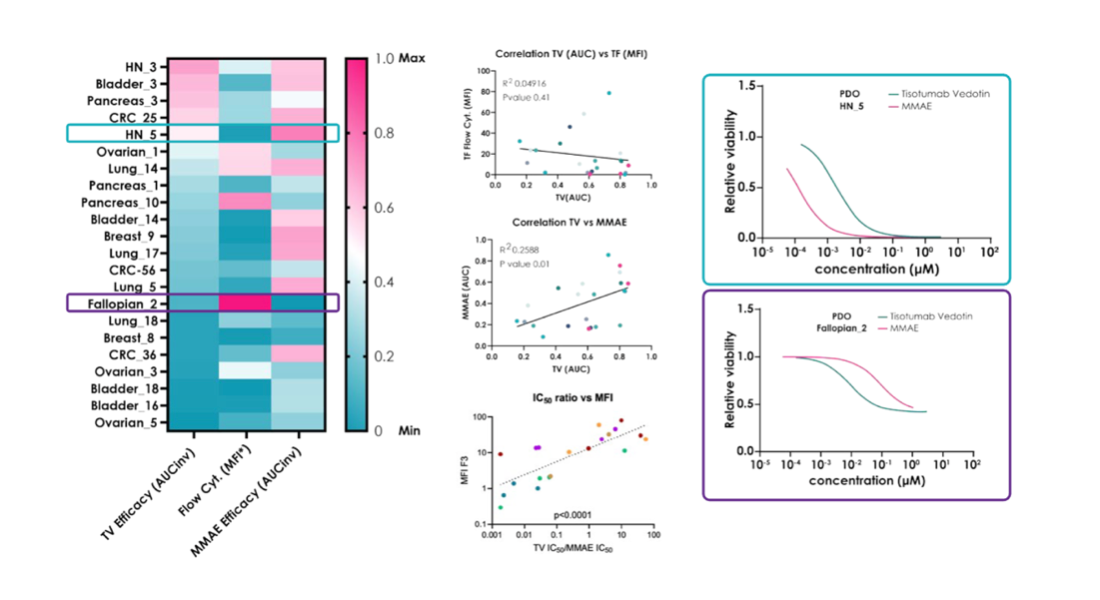
CD142 target expression doesn’t correlate with TivdakTM efficacy, but payload sensitivity does
So what was driving the difference? The organoid data pointed us toward payload sensitivity. Tumor cells varied widely in how they responded to the MMAE payload, regardless of how much CD142 they expressed on their surface. That finding suggested that intrinsic biological differences, not just target abundance, shape how effective an ADC will be.

CASE STUDY
How we supported our client in advancing biomarker discovery and translational ADC development
Rethinking ADC optimization
Conventional cell lines rarely capture the heterogeneity seen in patients. They can overemphasize target expression while masking differences in payload sensitivity, drug metabolism, and stress-response pathways. By contrast, organoids preserve tumor complexity, allowing researchers to see how a payload actually performs in a realistic tumor context.
Our findings emphasize that binding metrics alone are no longer enough to predict success. True optimization requires integrating target engagement with payload biology and organoids offer the most effective preclinical platform to achieve that.
Ready to Unlock True ADC Insights?
Discover how our ADC Screen can help you bring patient insights into your ADC preclinical drug development
