Forecasting a champion: How HUB Organoids® can speed up the drug development timeline to about five years
Published by Dnyanada Sahasrabudhe on May 17, 2022
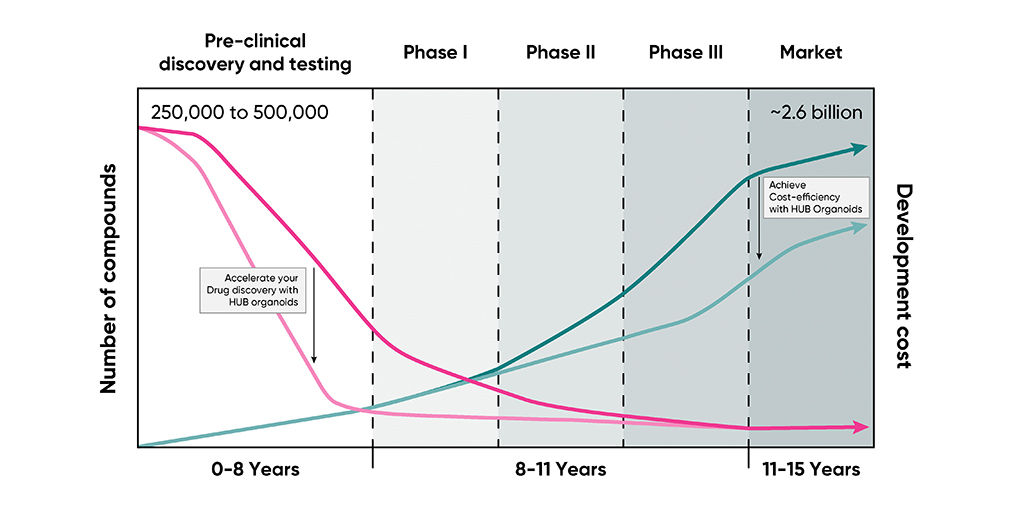
Learn why you should invest in patient-derived HUB Organoids® for your drug screening program
Drug discovery is like horse betting, except it takes 12 years to reveal the race's outcome. When the race is this long, an array of evidence-based estimations should be the go-to strategy to pick a winning candidate.
Betting on the right drug can be excruciatingly complex. The number of horses can scale up to 10,000 compounds, and the type of qualifying races can vary to account for clinical relevance. Since this race affects human life, it demands to be devoid of side effects or toxicities. In Oncology, where only 5% of anti-cancer agents tested in preclinical discovery and development make it to the Clinical Phase, this complexity translates to high attrition rates. Experts attribute this to the over-reliance on animal models or cell lines which only partially recapitulate the genetic and physiological intricacies of the patient tumors.
A leap towards patient-derived systems
In 2016, the US National Cancer Institute (NCI) acknowledged this by launching an initiative to retire its panel of human cancer cell lines, also called NCI-60, for drug screening purposes. The industry promoted the usage of patient-derived xenografts (PDX), which are created by implanting a small chunk of a patient tumor into immunosuppressed mice. PDX models garnered media attention as a possible avenue to cut down the losses earlier in the drug development pipeline by accounting for patient-relevant disease profiles and are still considered the gold standard preclinical models for translational oncology. Albeit better representative of patient physiology, PDX models are slow and labor-intensive to generate. Besides, as the stromal component of PDX models is not human, the effect of the microenvironment on drug response is not reflected. Furthermore, as PDX models are generated in mice with a compromised immune system, therefore the effect of any immunomodulatory drug cannot be evaluated.
The age of HUB Organoids
HUB Organoids are adult stem cell-derived mini-organs developed from patient resected or biopsied tissue that can be grown in the laboratory. HUB Organoids can retain the patient tumor's key genetic and physiological characteristics, are genetically stable, and can be easily expanded in the lab for drug screening. In addition, HUB Organoids can be generated from both the normal and malignant tissue of the same patient, thereby providing a unique opportunity to test for both antitumor efficacy and off-target toxicities. HUB Organoids take about 4-12 weeks to develop, compared to 6 months on average for a PDX and are gradually gaining traction as a better alternative to canonical systems such as cell lines or animal models.
All these characteristics and properties are interesting, but you may ask, is there any proof-of-concept that points to its role in accelerating drug development?
Developing a lead agent from early-stage discovery to clinical trials within five years
A proof-of-concept study recently published in Nature Cancer represents the first peer-reviewed published work in the oncology field that demonstrates the feasibility of using HUB Organoids to screen a library of compounds and progress a lead agent from early-stage discovery to clinical trials. The lead identified robustly blocks the growth of colorectal cancer (CRC) organoids with no toxicity effects observed on normal-tissue organoids derived from the same patient, thus validating its therapeutic potential in a clinical setting. Furthermore, the study demonstrates the effect of the lead on reducing tumor size and delaying metastasis in PDX models of colorectal cancer. Notably, the progression of this lead agent to the clinic was accomplished in about five years, a significantly shorter timeframe than a classic drug discovery and development pipeline. This promising agent has entered clinical trials. The preliminary reports from the Phase I dose-escalation studies indicate that the antibody is well-tolerated by patients with manageable infusion-related reactions and has shown promising results in five head and neck cancer patients by reducing their tumor size. (ClinicalTrials.gov identifier: NCT03526835).
The future ahead
This revolutionary proof-of-concept study demonstrates that it is possible to develop better, more effective anticancer agents in a much shorter timeframe using a clinically relevant, patient-derived system such as HUB Organoids in large-scale screening.
It takes 10-15 years and an average of US$1billion to develop a successful drug. Wouldn't you bet on a strategy that enables you to make faster, more cost-efficient, and clinically relevant estimates when the stakes are this high?
Want to use organoids for your drug development programs? Click here to know more.

.png)
-1.png)